
Contents of a Hazard Analysis Critical Control Point (HACCP) Plan. O Example of consumer advisory and letter of guarantee from seafood/fish supplier. HACCP Plan Template for Acidification of Rice in complaince with California Retail Food Code Requirements SAMPLE #1 Nigiri/Maki Roll (Raw Fish) and Sushi Rice HACCP. HACCP Plan – Slaughter. Supplier may provide a letter of guarantee stating that the hazard should not be present. On the other hand, a history of outbreaks or contamination related to a hazard would mean that the hazard IS reasonably likely to occur.
As governmental oversight and enforcement in the US becomes stricter and potentially more costly for US importers, wholesalers and retailers, foreign manufacturers will be asked to vouch for the safety and label compliance of their exported products.
That’s why more and more industrial and retail customers in the US request a legally binding Letter of Guarantee or and Affidavit from their foreign suppliers that they do not ship adulterated or misbranded food – as defined by the Food & Drug Administration.
Similarly, the increasing wave of lawsuits in California due to non-compliance with Prop 65, or the potential wave of lawsuits in Vermont, due to non-compliance with the Non-GMO law C.P. 121
Here’s the text for the Letter of Guarantee recommended by the authorities:
Letter of Guarantee: Safety
(1) Limited form for use on invoice or bill of sale (i.e. limited to a specific shipment of an article, in which case it may be a part of or attached to the invoice or bill of sale covering such shipment)
(Name of person giving the guaranty or undertaking) hereby guarantees that no article listed herein is adulterated or misbranded within the meaning of the Federal Food, Drug, and Cosmetic Act, or is an article which may not, under the provisions of section 404, 505, or 512 of the act, be introduced into interstate commerce.
(Signature and post-office address of person giving the guaranty or undertaking.)
(2) General and continuing form (considered to have been given at the date such article was shipped or delivered by the person who gives the guaranty)

The article comprising each shipment or other delivery hereafter made by (name of person giving the guaranty or undertaking) to, or in the order of (name and post-office address of person to whom the guaranty or undertaking is given) is hereby guaranteed, as of the date of such shipment or delivery, to be, on such date, not adulterated or misbranded within the meaning of the Federal Food, Drug, and Cosmetic Act, and not an article which may not, under the provisions of section 404, 505, or 512 of the act, be introduced into interstate commerce.
(Signature and post-office address of person giving the guaranty of undertaking.)

Letter of Guarantee: color additives for foreign manufacturers:
(Name of manufacturer and agent) hereby severally guarantee that all color additives listed herein were manufactured by (name of manufacturer), and (where color additive regulations require certification) are from batches certified in accordance with the applicable regulations promulgated under the Federal Food, Drug, and Cosmetic Act.
(Signature and post-office address of manufacturer.)
(Signature and post-office address of agent.)
Letter of Guarantee; GMO

As the Vermont law Consumer Protection Rule # 121 comes into effect July 1, 2016, US importers and their foreign suppliers may have to send to their customers the following sample Letter of Guarantee or Affidavit
I, ________________________, as the authorized agent of the manufacturer/producer listed above, hereby depose and state as follows:
The above named product(s) contains NO ingredients, additives or processing aides derived from commodities or seeds that have commercially grown genetically engineered varieties in the supply chain (i.e. contains no soybean, corn, cotton, canola, beet sugar, squash or papaya) or have not been knowingly or intentionally produced with genetic engineering and has been segregated from and has not been knowingly or intentionally commingled with food that may have been produced with genetic engineering.
I declare or affirm, under penalty of perjury, that the above statement is true and correct to the best of my knowledge.
Alternative:
The above named product(s) contain ingredients, additives or processing aides derived from commodities that have commercially grown genetically engineered varieties in the supply chain, BUT do not contain material derived from genetically modified plants because they have been sourced from traditional crops under an identity preserved program that is documented by the supplier.
Haccp Letter Of Guarantee Templates Free
It may help foreign manufacturers to obtain a Non-GMO certification, if this will help to sell and market their products in the US. The most widely recognized NON-GMO seal on food products comes from the Non-GMO Project.
FSMA: The Meaning of Misbranded Foods
California Proposition 65
Vermont Non-GMO law
FSMA: The Meaning of Adulterated Foods
Application of the Principles of HACCP
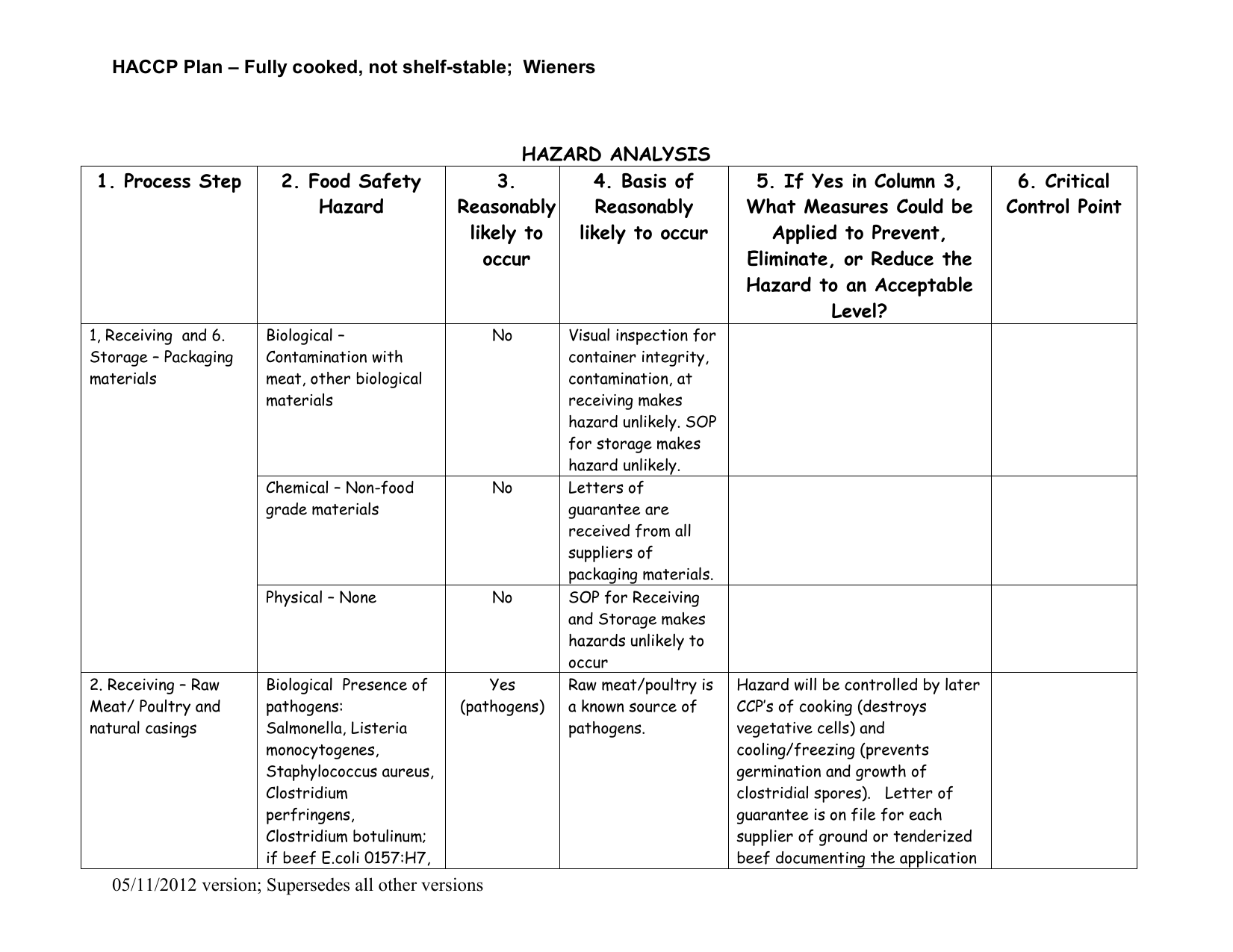
Principle 1 - Conduct a Hazard Analysis
The application of this principle involves listing the steps in the process and identifying where significant hazards are likely to Occur. The HACCP team will focus on hazards that can be prevented, eliminated or controlled by the HACCP plan. A justification for including or excluding the hazard is reported and possible control measures are identified.
Principle 2 - Identify the Critical Control Points
A critical control point (CCP) is a point, step or procedure at which control can be applied and a food safety hazard can be prevented, eliminated or reduced to acceptable levels. The HACCP team will use a CCP decision tree to help identify the critical control points in the process. A critical control point may control more that one food safety hazard or in some cases more than one CCP is needed to control a single hazard. The number of CCP's needed depends on the processing steps and the control needed to assure food safety.
Haccp Letter Of Guarantee Templates Pdf
Principle 3 - Establish Critical Limits
A critical limit (CL) is the maximum and/or minimum value to which a biological, chemical, or physical parameter must be controlled at a CCP to prevent, eliminate, or reduce to an acceptable level the occurrence of a food safety hazard. The critical limit is usually a measure such as time, temperature, water activity (Aw), pH, weight, or some other measure that is based on scientific literature and/or regulatory standards.
Principle 4- Monitor CCP
The HACCP team will describe monitoring procedures for the measurement of the critical limit at each critical control point. Monitoring procedures should describe how the measurement will be taken, when the measurement is taken, who is responsible for the measurement and how frequently the measurement is taken during production.
Principle 5 - Establish Corrective Action
Corrective actions are the procedures that are followed when a deviation in a critical limit occurs. The HACCP team will identify the steps that will be taken to prevent potentially hazardous food from entering the food chain and the steps that are needed to correct the process. This usually includes identification of the problems and the steps taken to assure that the problem will not occur again.
Principle 6 - Verification
Those activities, other than monitoring, that determine the validity of the HACCP plan and that the system is operating according to the plan. The HACCP team may identify activities such as auditing of CCP's, record review, prior shipment review, instrument calibration and product testing as part of the verification activities.
Haccp Letter Of Guarantee Templates Letter
Principle 7 - Recordkeeping
A key component of the HACCP plan is recording information that can be used to prove that the a food was produced safely. The records also need to include information about the HACCP plan. Record should include information on the HACCP Team, product description, flow diagrams, the hazard analysis, the CCP's identified, Critical Limits, Monitoring System, Corrective Actions, Recordkeeping Procedures, and Verification Procedures.
Haccp Letter Of Guarantee Templates Printable
HACCP Does not Stand Alone
Haccp Letter Of Guarantee Template
The application of HACCP does not stand alone in a food processing facility. The plan must be built on other food safety programs. Good Manufacturing Practices (GMP) that are practiced by the processing facility will support HACCP plan and will address food safety and food quality issues that are not critical for the reduction of food safety hazards. Sanitation Standard Operating Procedures (SSOP's) are required in federally inspected meat and poultry operations and address procedures for clean facilities, equipment and personnel that are necessary for all products produced in a facility.